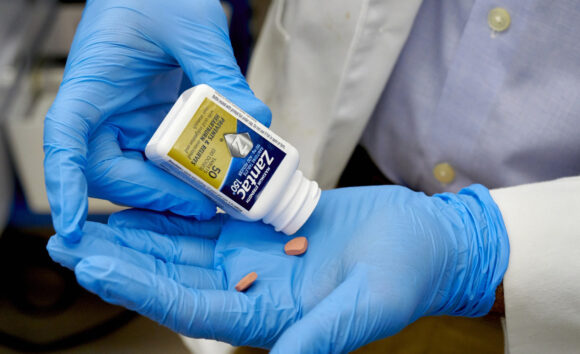
Sanofi will pay more than $100 million to settle about 4,000 lawsuits accusing the French drugmaker of failing to warn users that its Zantac heartburn medicine could cause cancer, according to people familiar with the deal.
The settlement was announced earlier this month, but the amount was not made public at that time. The deal provides former Zantac users with an average of more than $25,000 per claim, said the people, who requested anonymity because they weren’t authorized to speak publicly.
The relatively modest settlement should please Sanofi investors, who’ve worried in the past about the financial fallout of thousands of US lawsuits targeting Zantac makers such as Sanofi and GSK Plc, which originally developed the product.
However, Sanofi’s settlement mainly applies to state court cases outside Delaware, where a judge is considering whether to allow the scientific evidence underlying more than 70,000 suits. A federal judge in Florida threw out more than 5,000 suits in 2022, saying the science behind the cancer claims was flawed.
Shares in Sanofi was up slightly in Paris trading on Monday. GSK was up around 2% in London.
Sanofi declined to comment on “speculation and rumors” about the settlement amount and denied that Zantac causes cancer. “Sanofi is settling these cases, not because we believe the claims have any merit, but rather to avoid the expense and ongoing distraction of the litigation,” a Sanofi spokesperson said.
Concerns about exposure to the Zantac cases helped wipe out about $45 billion in market value from GSK and Sanofi’s stock in the summer of 2022. The shares have since recovered. Analysts say the companies still face billions in potential damages, however.
GSK has settled a number of Zantac suits in California for terms that weren’t disclosed. One case against the UK drugmaker is likely to make it to a jury is set to start April 30 in state court in Chicago. Angela Valadez, 89, contends she used the heartburn treatment for nearly 20 years before being diagnosed with cancer. GSK and Boehringer Ingelheim GmbH, another company that made and sold Zantac, are the defendants in the case.
Zantac hit the US market as a prescription drug in 1983 before becoming an over-the-counter heartburn treatment in 1996. It was owned by several companies, including Pfizer Inc. and Boehringer before Sanofi acquired it in 2017. Former users allege the companies knew Zantac posed a cancer risk and sold it anyway without warnings. Makers of a generic version of the drug also are being sued.
Zantac was recalled in 2019, about a month after an independent lab announced publicly it found the likely carcinogen NDMA in the drug. The lab’s research indicated Zantac’s active ingredient at the time — ranitidine — formed NDMA over time or in elevated temperatures. US regulators confirmed the findings in April 2020 and ordered the medicine off the market.
Sanofi has since won approval to return Zantac to US store shelves, but without ranitidine. It’s now made with famotidine, the active ingredient in competitor Pepcid. Zantac is still one of the US’s leading heartburn treatments in a market that generated $14 billion in sales last year.
Top photo: A senior research associate prepares to test a bottle of Zantac 150.
Was this article valuable?
Here are more articles you may enjoy.

 Hackers Hit Sensitive Targets in 37 Nations in Spying Plot
Hackers Hit Sensitive Targets in 37 Nations in Spying Plot  LA County Told to Pause $4B in Abuse Payouts as DA Probes Fraud Claims
LA County Told to Pause $4B in Abuse Payouts as DA Probes Fraud Claims  US Will Test Infant Formula to See If Botulism Is Wider Risk
US Will Test Infant Formula to See If Botulism Is Wider Risk  Berkshire Utility Presses Wildfire Appeal With Billions at Stake
Berkshire Utility Presses Wildfire Appeal With Billions at Stake 