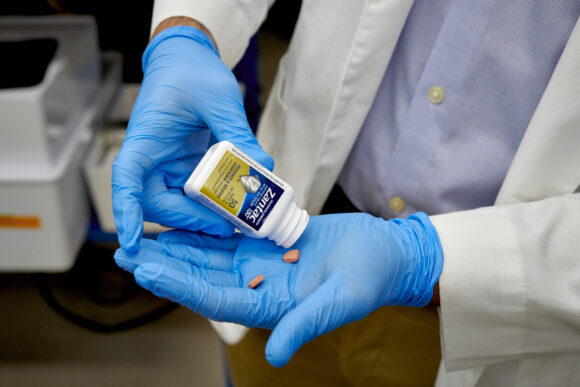
GSK Plc convinced an Illinois jury that the former heartburn drug Zantac was not liable for a woman’s colorectal cancer in a second trial win for the British drugmaker.
It comes just over two months after jurors in Chicago also found that GSK and Boehringer Ingelheim GmbH weren’t liable for another woman’s illness.
The company’s share price rose slightly in early London trading. GSK stock is up nearly 7% since the start of the year.
Related: GSK Settles Another Heartburn Drug Lawsuit in Illinois
GSK is one of several drugmakers facing lawsuits from people who argue the companies knew ranitidine — Zantac’s active ingredient — turned into the potential carcinogen NDMA under certain conditions. In 2020, the US Food and Drug Administration asked companies to remove all ranitidine-based drugs from the US market.
The verdict in Illinois is “consistent with the scientific consensus that there is no consistent or reliable evidence that ranitidine increases the risk of any cancer,” GSK said in a statement Tuesday.
GSK is still facing thousands of cases in the US. A judge earlier this year said that GSK, Pfizer Inc. and others must face trials in a Delaware state court.
Related: Industry Groups Back Drugmakers’ Appeal in Zantac Cancer Lawsuits
GSK has already settled several cases, while not admitting liability.
The Zantac litigation has overshadowed progress GSK has made elsewhere, including with its vaccine for a common respiratory virus that outpaced sales of competitor Pfizer’s shot in the US.
Under Chief Executive Officer Emma Walmsley, GSK has worked to prioritize products in areas where it has existing strengths such a vaccines. Walmsley also demerged GSK’s consumer health business, enabling the company to focus on innovative new medicines.
Was this article valuable?
Here are more articles you may enjoy.

 One out of 10 Cars Sold in Europe Is Now Made by a Chinese Brand
One out of 10 Cars Sold in Europe Is Now Made by a Chinese Brand  UBS Top Executives to Appear at Senate Hearing on Credit Suisse Nazi Accounts
UBS Top Executives to Appear at Senate Hearing on Credit Suisse Nazi Accounts  Elon Musk Alone Can’t Explain Tesla’s Owner Exodus
Elon Musk Alone Can’t Explain Tesla’s Owner Exodus  Hackers Hit Sensitive Targets in 37 Nations in Spying Plot
Hackers Hit Sensitive Targets in 37 Nations in Spying Plot 